Casual Info About How To Detect Electrons

Thus, the electron configurations for the next six atoms are as follows:
How to detect electrons. Calcium’s atomic number is 20. The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1 s2 2 s2. If the charge is positive, the ion has lost electrons.
I have a webapp which will run on a website and as a standalone electron instance (.exe for windows). Mit physicists have developed a new tabletop particle detector that is able to identify single electrons in a radioactive gas — and may one day help measure neutrino mass. As stated, the electron configuration of each element is unique to its position on the periodic table.
The periodic table is a chart that organizes elements by their atomic structure. For example, how many neutrons does an atom with a mass of 79 and 35 electrons have? Just as in a tem, the top of a.
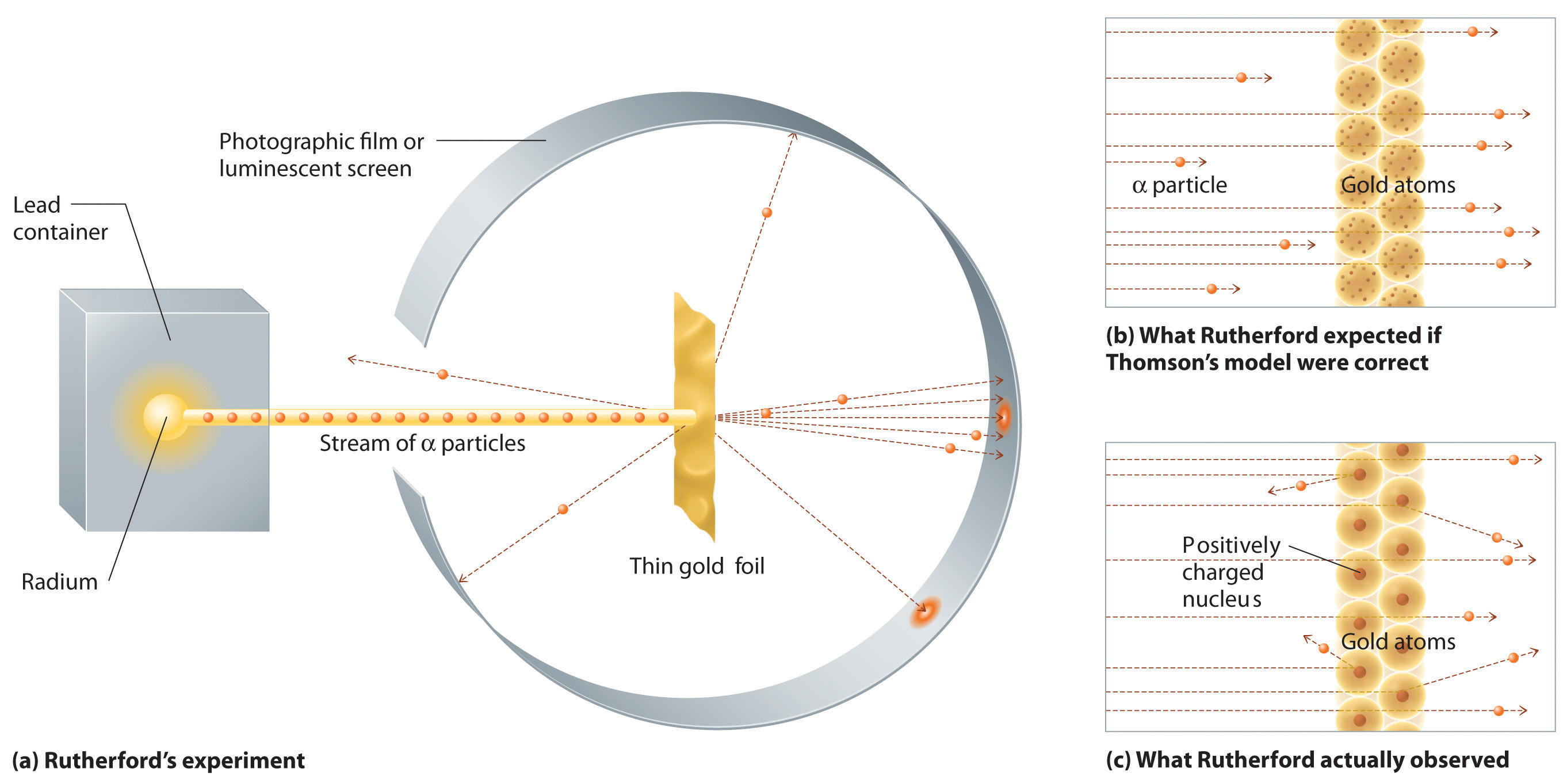
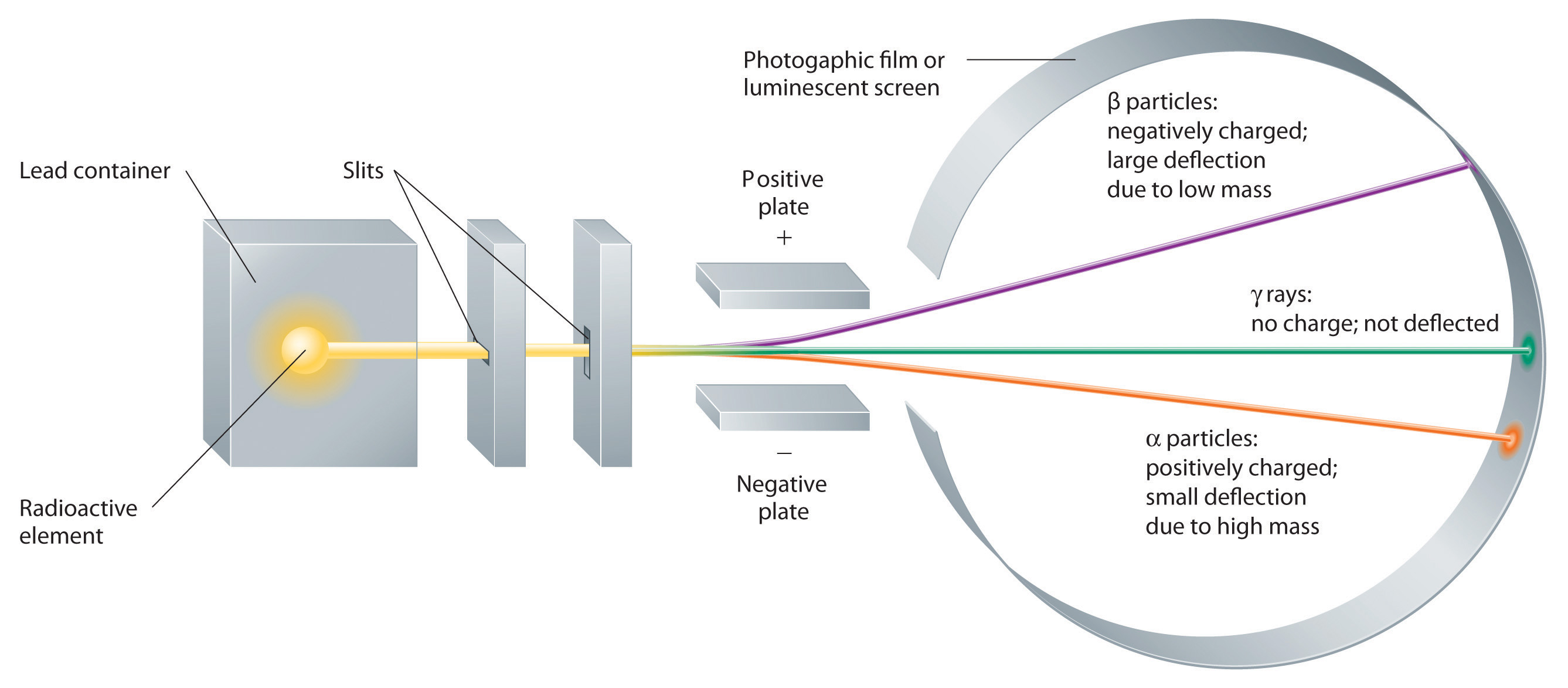
It also explains the differe. Find your element on the periodic table. It is usually designed to stop entirely or “absorb” most of the particles coming from a collision, forcing them to deposit all of their energy within the detector, thus measuring their full.
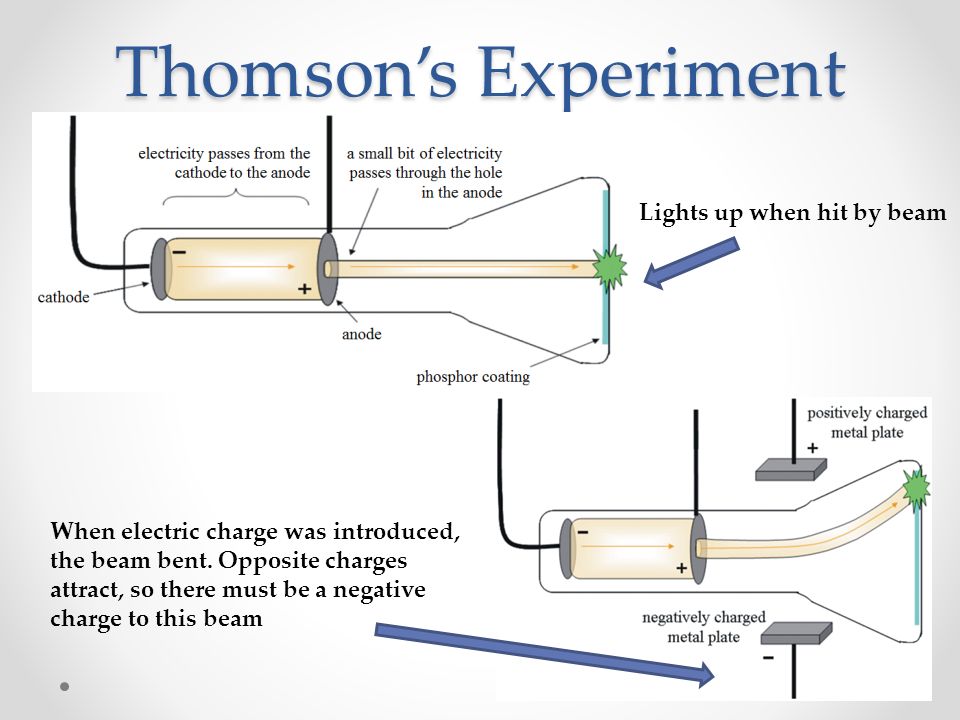
This research was funded in part by the department of energy and the national science foundation. These interactions can produce rare electronic states, such as the seemingly unorthodox splitting of an electron’s charge. That interaction can be used to provide information about compounds containing unpaired electrons.
The periodic table reveals lots of information about the elements — we'll use some of this information to determine the number of valence electrons in the atom we're investigating. One of the hallmarks of unpaired electrons in materials is interaction with a magnetic field. The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square.
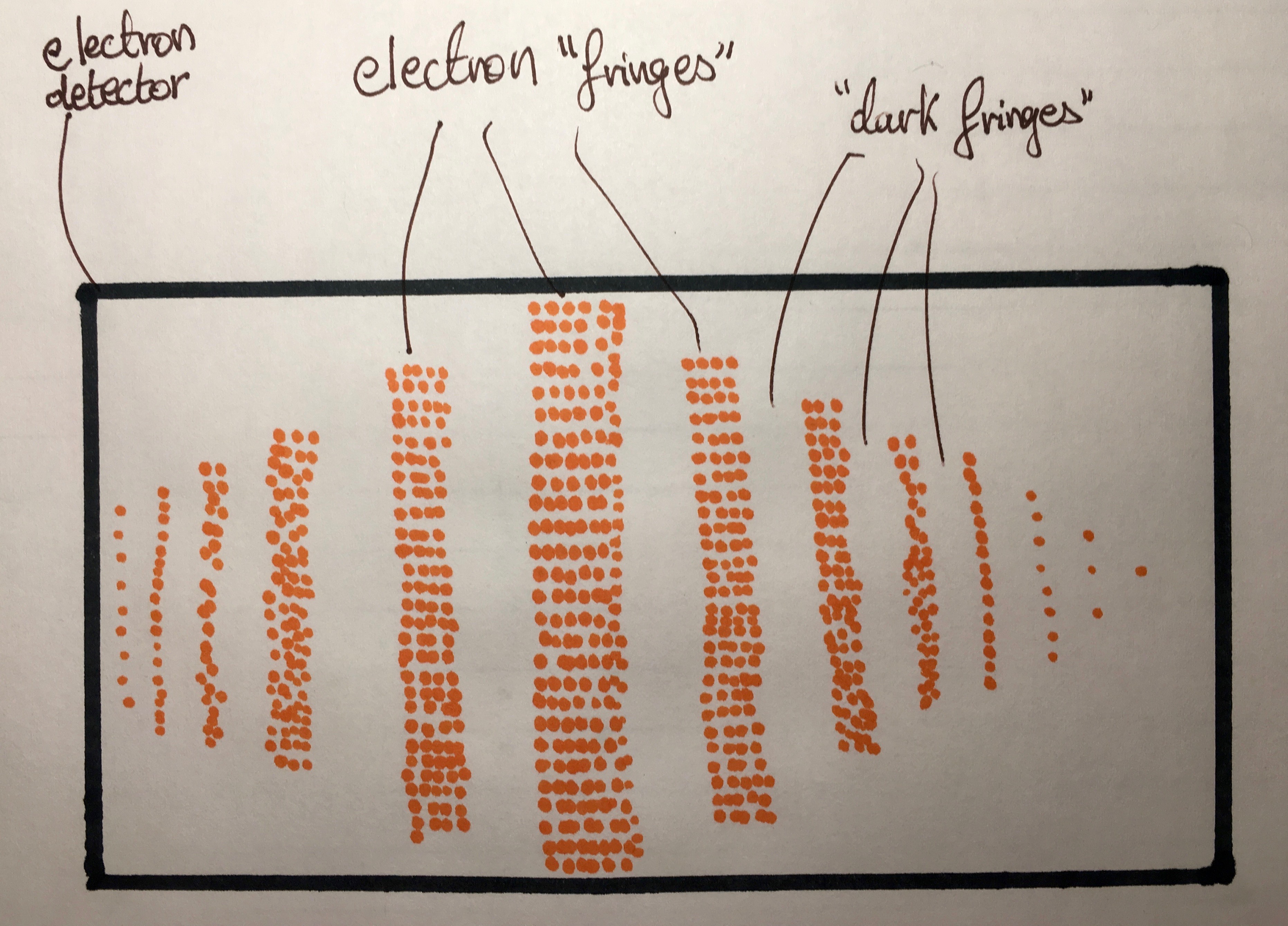
Shell diagram of lithium (li) atom. This chapter will explain how we ‘see’ electrons. We can therefore calculate the number of moles of electrons transferred when a known current is passed through a cell for a given period of time.
The p, d, and f orbitals have different sublevels, thus can hold more electrons. Figure 2.4.1 2.4. 1 find a periodic table of elements.
For example, ca 2+ has a +2 charge, therefore, it has 2 fewer electrons than a neutral calcium atom. This chapter will explain how we ‘see’ electrons. Scanning electron microscopes (sems) most of the funky electron microscope images you see in books—things like wasps holding microchips in their mouths—are not made by tems but by scanning electron microscopes (sems), which are designed to make images of the surfaces of tiny objects.
Think of this as knowing not only which apartment buildin. Modified 3 years, 9 months ago. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one.